
In the first part of the reaction, the biotin cofactor is carboxylated using bicarbonate as a substrate with ATP to drive the formation of a "high energy" bond with respect to its hydrolysis product intermediate. Each subunit contains a biotin carboxylase (BC) domain shown in blue, a carboxytransferase (CT) domain shown in yellow, and the biotin-carboxyl carrier protein (BCCP) domain shown in red and green.įigure modified from Liu, Y., et al (2018) Nat Commun 9:1384 (a) shows the space-filling model while (b) shows the major domains as a cartoon graphic.

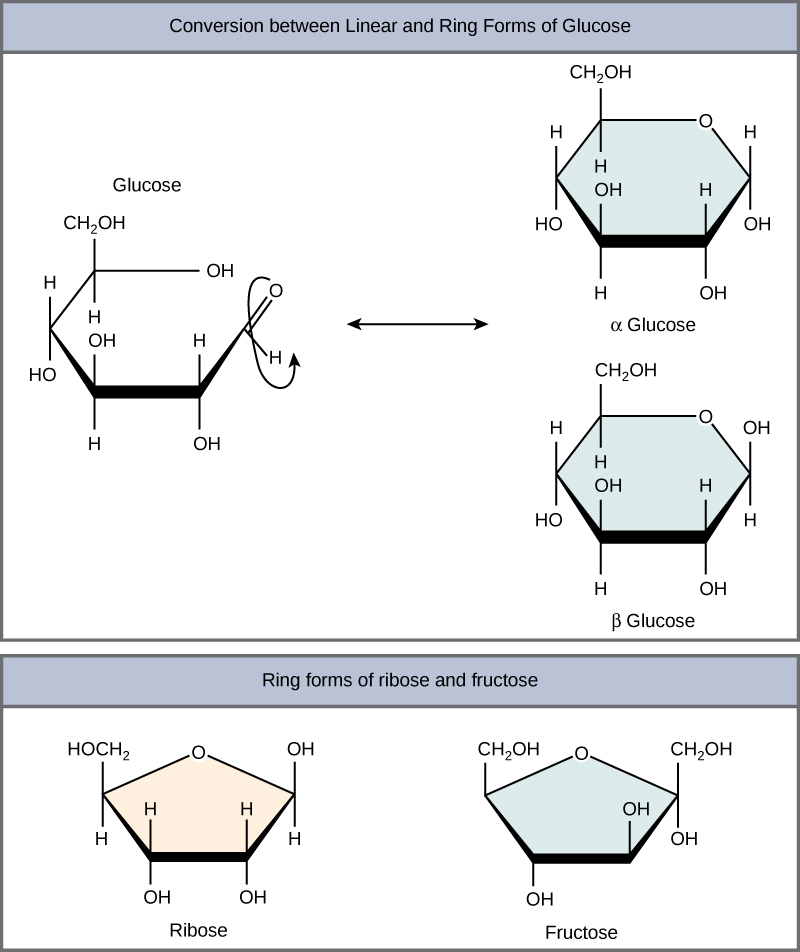
Two subunits are shown in color, with the other two indicated in gray at the back of the structure. The overall structure of the Pyruvate Carboxylase is a tetramer that contains four functional protein subunits. \) Structure of the Pyruvate Carboxylase Enzyme. These differences affect the properties of the two monosaccharides.


 0 kommentar(er)
0 kommentar(er)
